Group News
Predicting synthesizability of crystalline materials via deep learning
Sara Kadkhodaei, an affiliated faculty member of CDAC and co-PI of DMREF, is co-authored on a paper titled, “Predicting synthesizability of crystalline materials via deep learning” in Nature publishing journal Communications Materials! See the paper here.
Abstract
Predicting the synthesizability of hypothetical crystals is challenging because of the wide range of parameters that govern materials synthesis. Yet, exploring the exponentially large space of novel crystals for any future application demands an accurate predictive capability for synthesis likelihood to avoid a haphazard trial-and-error. Typically, benchmarks of synthesizability are defined based on the energy of crystal structures. Here, we take an alternative approach to select features of synthesizability from the latent information embedded in crystalline materials. We represent the atomic structure of crystalline materials by three-dimensional pixel-wise images that are color-coded by their chemical attributes. The image representation of crystals enables the use of a convolutional encoder to learn the features of synthesizability hidden in structural and chemical arrangements of crystalline materials. Based on the presented model, we can accurately classify materials into synthesizable crystals versus crystal anomalies across a broad range of crystal structure types and chemical compositions. We illustrate the usefulness of the model by predicting the synthesizability of hypothetical crystals for battery electrode and thermoelectric applications.
Novel Li-F-H Compounds
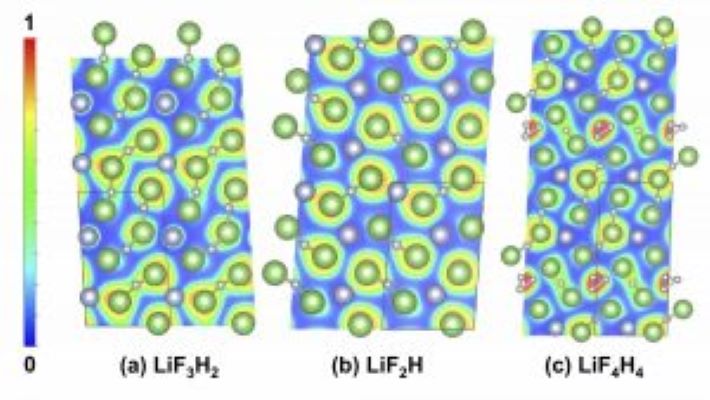
A paper from CDAC involvng teams at Buffalo, UIC, and Livermore have uncovered new pressure-induced chemistry associated LiF and hydrogen, results that have implications for dynamic compression experiments involving these materials.
Possible pressure-induced chemical reactions between hydrogen and LiF windows in dynamic compression experiments near 300 GPa44, could affect the interpretation of the results. Given the propensity for formation of stable and metastable hydrides under pressure, we were motivated to apply crystal structure prediction techniques to the Li-F-H ternary system to multimegabar pressure. By now the phase diagrams of the elemental (Li, H, and F) and binary (Li-H, H-F, and Li-F,) systems have been studied computationally up to 300 GPa, providing the basis for us to explore the high pressure phases of the ternary system.
Evolutionary crystal structure prediction techniques were used by the team to explore ground state structures containing the elements Li, F, and H at pressures. None of the structures found suggest compound formation in the NIF experiments using LiF and hydrogen isotopes. However, a number of intriguing metastable phases are predicted that could be synthesizable.
Common structural motifs present in these phases include HnF−n+1 anions of various lengths and Li+ counter-cations. Most of these crystalline lattices are calculated to be wide-gap insulators, with the exception of LiF3H, which is predicted to be metallic and superconducting below 0.1 K. Li3 F4 H is found to be thermodynamically and dynamically stable at atmospheric pressure.
Exploratory calculations on the LiF4H3 stoichiometry predict a structure that contains bent and asymmetric bifluoride anions, as well as infinite HF chains with nearly equal bond lengths, whose enthalpy lies 21.7 meV/atom above the convex hull. Moreover, Li3 F4 H was predicted to be thermodynamically and dynamically stable at zero pressure, suggesting that other Li-F-H compounds with unique HnF−n+1 could be synthesized and stabilized at ambient conditions. This studies provides the basis for fu- ture work exploring the finite temperature stability of Li-F-H phases, with the inclusion of anharmonic effects, which are known to be important for light element systems especially at high pressures.





